A while back, I read a very good CQ January 2019 article by Klaus Spies WB9YBM on “A Better Battery Charger for HTs.” One statement in the article caused me to contact Klaus: “Yes, this battery type (NiMH) is still susceptible to developing a memory ...”
On this point, Klaus and I disagree because of our different experiences. I have worked for two companies. In one, I was the “battery expert” and the other I helped someone else dealing with rechargeable batteries because of my previous experience.
When I started working with rechargeable batteries at Gardner Laboratory, I read every article I could find about the so-called “memory effect” of NiCd batteries. Nothing I found in the literature of the time was able to confirm the existence of this effect, and in my subsequent experience, I have not yet come across a battery pack that I could clearly say exhibited this effect.
This doesn’t mean that the effect doesn’t exist. It simply means that I haven’t seen it. So, let’s say it does exist. The question then becomes, why haven’t I seen it in all the battery packs I’ve taken apart? I believe that a battery pack with a real memory effect is far less common than most people think. None of the packs thought to have been exhibiting a memory effect that I have taken apart to test have actually had any battery with a memory effect.
I have found all sorts of failure modes and I’ll discuss the most common failures I’ve experienced and been able to confirm. Note, all of these failure modes may look — on first blush — like the memory effect failure mode, but after some testing the conclusion was that there was no memory effect.
Let’s try to define a memory effect. It’s said to be caused by continually discharging a battery pack to some intermediate level of discharge and then recharging it. This level of discharge comes before the full discharge of the pack.
It exhibits a “memory” of this level of discharge and will not discharge any lower, thus limiting the battery pack to that less-than-full-discharge level.
We now have a working definition to test against.
NASA is said to have been the first to experience this memory effect on one or more of its satellites.
There are three reasons I don’t believe I’ve ever seen this memory effect:
- I believe that it’s almost impossible — under normal use — that we (as users) will ever be able to stress our battery packs to exactly the same extremely regular charge/discharge regimen that is suspected to cause the memory effect. (I know of at least one possible exception to this statement. If we don’t use our device and recharge it at some very regular interval [say, every 60 days] to keep the pack fully charged and we do so at exactly the same time each time, this might fall into this definition and might possibly cause a memory effect in the battery pack.)
- We can inadvertently kill the memory effect simply by using our packs until the device it’s in no longer works. For our radios, we can no longer transmit or receive. Here, we have inadvertently done exactly what is recommended to rejuvenate a memory-effected battery pack.
- Normal degradation will eventually make the pack look like the memory effect because the whole pack eventually degrades to the point that it can no longer be used for the same amount of time compared to when it was new.
Let’s leave the memory effect behind at this point and concentrate on the failure modes I’ve come across. Many of these can make a pack of batteries look like it’s suffering from a memory effect. The difference is seen when the pack is taken apart and each battery/cell is tested and/or recharged individually.
The first problem I noticed with NiCd battery packs was that after a few years they would not hold a charge for as long as they did originally. Sure looked like a memory effect. To find out what was causing this, I took the battery packs apart and tested each individual battery. These were all AA NiCd batteries soldered together as a pack of six or eight cells, thus making 7.2 or 9.6 volt battery packs.
The most common failure that I noticed was that one (or more) of the individual cells was much weaker than all the others. If this problem was caught soon enough and the battery had not been reverse charged, I was able to charge the cell (or cells) individually and bring them back up to the same voltage and charge level as the other cells in the pack.
Once this was done, the pack would give at least another year of good service close to the original level of the new battery pack. When I had such a pack, I would take it apart every year to recharge all the cells individually. These packs — with each battery recharged individually every year — would last up to about 10 years! That leads me to my present type of battery packs for my radios, described below.
The second failure mode I came across was caused by a reversed charged battery. If the individual cell (or cells) were reverse charged, they had to be removed and replaced with other new cells. In my experience, this was never a good solution because the bad cells were being replaced with new cells.
This pack was now made up of a few new cells where all the other cells were still three or more years old. This meant that the new cells would fully charge a bit sooner and hold their charge a bit longer than the older cells. This put extra stress on the older cells and caused them to die sooner than they would have if all of the cells had been the same age.
The main difficulty in replacing individual cells is this age and quality difference. NASA could stipulate and pay for an extremely tight specification on the charge/discharge characteristics on each cell/battery of the battery packs they received. The rest of us could not afford to pay for this kind of testing and level of matching.
Because of this, our packs normally last about three or four years of regular use, while the battery packs in satellites need to last 10+ years of very regular daily charge and discharge cycles. We’re talking about 10 years of 365 cycles every year for a total of 3,650 charge/discharge cycles. Imagine if our packs would last for 3,650 cycles. This would mean that if they needed to be charged every two months — used or not — they would last for about 608 years.
I don’t know if NASA now has a battery reconditioning mode built into its satellites, but one can assume that if the memory effect is a problem for a satellite, then they’ve found some way around it. We, too, can recondition our packs every year or so to keep them in tip top shape.
When a pack showed one or more cells reverse charged, it was best to replace the battery pack with one built by a reputable replacement battery pack dealer.
This then was my solution to continually having to buy high priced rechargeable battery packs: I found that it made more sense to buy empty battery cases that would accept AA batteries, and then use NiCd or NiMH batteries and charge them outside of the case individually.
I had NiCd batteries for over 10 years that still worked well for me using this method. I now use Eneloop NiHM batteries. These don’t have the highest capacity, but the newer Eneloop batteries claim a shelf life of 70% of full charge after 10 years on the shelf. The original Eneloop batteries only claimed 70% after three years on the shelf.
When I discovered these around six years ago, I replaced all my NiCds and original NiMH batteries with these NiMH long shelf life batteries. I also replaced all my chargers with ones that charge each cell individually. You can find these on eBay that claim a five year shelf life of 70% of full charge now. For emergency service, I felt that long shelf life was much more important than an extra 500 mAh or so capacity. If these did as claimed, I could confidently pick up my go-bag of radio and two battery packs and know I could operate for almost 90% of newly charged batteries if I made sure to charge these batteries once a year.
The third most common failure mode I came across in these battery packs was that one or more cells were “dead,” measuring zero volts and not taking a charge at all. This was caused by dendrites. These are very small growths inside the battery that act like wires short-circuiting the battery so that it not only measures zero volts, but it shorts out the charger and simply can’t take a charge.
I read that if the battery is hit with a very high voltage for a short duration, it will clear the dendrites and the battery would function once again. I tried this with all the batteries that exhibited this type of failure mode but the results were not as hoped.
In my experience with this type of failure, hitting the battery with a high voltage for a short duration did, in fact, clear the dendrites. The battery then not only measured the correct voltage but also functioned as a good battery — at least for a little while. The problem, as far as I could tell, was that once the battery came to this point, it was probably some kind of chemistry problem because batteries returned from the dead in this way would die again with more dendrites until there were probably so many dendrites that the battery was no longer able to be recovered.
I eventually came to the conclusion that so many dendrites are formed that even a high current/high voltage blast is handled by the number of dendrites and the battery is truly dead.
Once I figured this out, I simply recycled these batteries. It wasn’t worth my time and effort to go through this procedure over and over again until the battery could no longer be cleared of dendrites.
The fourth type of failure is a battery that charges up to the correct voltage but then drops to a much lower voltage when it’s put into normal operation. Again, this makes the full battery pack look like it suffers from a memory effect but, in fact, this is because — again — one or two of the batteries in the pack are “dead.” This particular type of dead battery is usually caused by dried out chemistry.
It can look like it’s fully charged, but the chemistry no longer exists to support the former current capability of the original battery. This is easy to determine and the battery should be recycled. There is no way to repair the chemistry of the battery.
The fifth failure mode is simply old age. All battery chemistries age, dry out, or there is anode and/or cathode degradation. These all cause less capacity than a new battery has. I have never seen this type of failure mode due to seeing the other four types listed above before accurred old age. To help get data, I put out a call to the Carroll County Amateur Radio Club (CCARC) membership to see if anyone had any Ni-Cd, Ni-MH, and/or Li battery packs which were dead that I could test and then take apart to see if I could figure out what went bad. I received four Ni-Cd packs and two Li packs. Unfortunately, all of these packs had the same kind of failure mode which I had never come across before. This was the fifth type of pack failure listed above.
The fifth type of failure is a battery pack that charges to the correct voltage and then works for a lesser amount of time than it originally did when new. This is the very definition of the memory effect. But — and here’s the caveat — this is also the expected failure mode of an older degraded battery. It simply gets old and the chemistry is failing, so the battery can only operate for a shorter amount of time. The difference between memory effect and older dying batteries is simply the age of the pack. The memory effect happens to batteries which are not yet old enough to be expected to have a significantly shorter operating time.
Time and age causes the normally expected degradation of all the batteries in the pack. Interestingly, I had not come across this type of failure before. Since I did all my original battery pack studies prior to my having an easy way to test the packs, I never bothered to record all the data for the packs and individual batteries. A few years ago I purchased a Computer Battery Analyzer (CBA) so I could test all my emergency batteries and know how good they were when first purchased. Then, if I suspected a battery failing, I could test it again and compare.
Ni-Cd Battery Tests
The first two packs I tested were actually from a combined pack for an NCR machine. When the large pack was taken apart, I found two smaller packs inside in series to form a 24V supply for the single pack amp-hour rating. Both packs held 10 Ni-Cd batteries to make a 24V @ 1.4 Ah pack. So, each pack was 12V and 1.4 Ah. Refer to Figures 1 and 2.

FIGURE 1. NCR battery packs taken apart.

FIGURE 2. NCR discharge setup using a CBA (computerized battery analyzer).
The first pack test was so bad I had to recharge the pack to even get a curve. The first test lasted less than 15 seconds; closer to five seconds. The second test — after 20 hours of charging — gave something that at least could be measured. The curve in Figure 3 is for both packs after a full recharge of 20 hours or so.
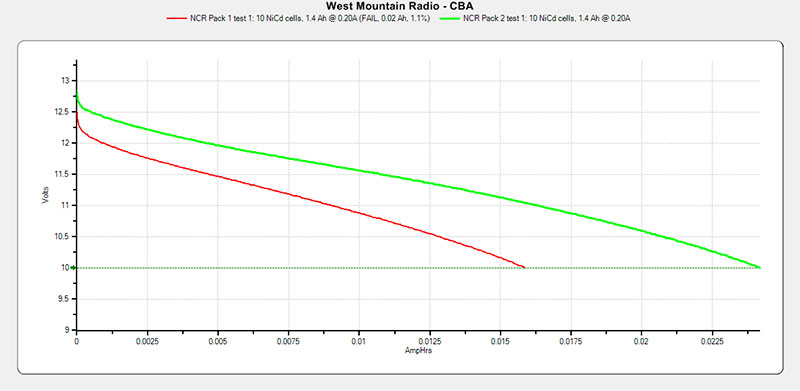
FIGURE 3. NCR pack test results.
Retest of Pack 1 again labeled as Test 1 and Pack 2 also recharged for its first test. Pack 1 = red curve; Pack 2 = green curve. It didn’t seem worthwhile to test Pack 2 until it had also been charged for at least 20 hours. So, the initial test of Pack 1 was ignored.
It can be seen that Pack 1 tested around 17 mAh and Pack 2 tested around 24 mAh for packs that were designed for 1,400 mAh (*1.4 Ah). These are as dead as you can get.
I didn’t try to charge these cells individually and test them again. I expect that if I had done that they would have improved to around 50 mAh, but not up to their original rating of 1.4 Ah. All the batteries were measured and found to have voltages within 0.02V of each other. These were extremely well-matched sets of batteries.
The next pack I tested was the Yaesu pack shown in Figure 4.

FIGURE 4. Yaesu FT-209 battery pack.
Figure 5 shows the pack opened.
FIGURE 5. Yaesu FT-209 battery pack (open).
Figure 6 shows the batteries out of the pack and numbered.

FIGURE 6. Yaesu FT-209 batteries out of their pack and numbered.
The first test only lasted about 10 seconds, so I figured that even though the overall voltage of the pack was correct, it had not been charged. The pack was then charged overnight. The second test was better but still very evident that the pack was dead, since the 600 mAh pack only tested at about 161 mAh. See the results in Figure 7.
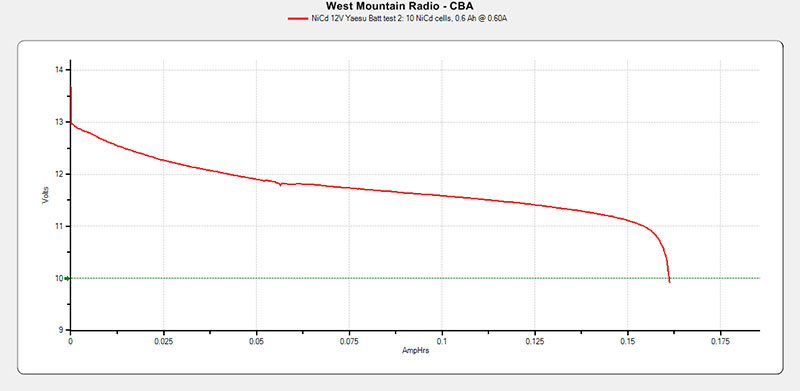
FIGURE 7. Yaesu NiCd 12V battery test results.
The voltages of all the cells were measured before the first test, after the first test, after the overnight charge, then just before the second test and again after the second test. All 10 batteries measured within 0.01V of each other every time they were measured.
Before the first test, they measured at 1.21 ±0.01V, and before the second test they measured 1.40 ±0.01V right after being taken off the charger. After a three-hour rest and just before Test 2 was run, they measured 1.37 ±0.01V.
On the Figure 7 graph, it can be seen that the initial voltage was about 13.6V. This voltage was taken as the test began with a 600 mA discharge, which would have been probably half of the drain the radio would put on the pack when transmitting at 5W.
The Kenwood PB-13 Pack
The last pack tested was the Kenwood PB-13 pack, shown in Figures 8 through 11.

FIGURE 8. Kenwood PB-13 battery pack (closed).

FIGURE 9. Kenwood PB-13 battery pack (open).

FIGURE 10. Kenwood PB-13 battery pack (top view).

FIGURE 11. Kenwood PB-13 batteries.
These batteries were all well below the recommended discharged voltage for Ni-Cd batteries ranging from 0.50 to 090 volts, so all batteries were charged individually for 5-8 hours at a constant current of 110 mA. The voltages on the batteries varied from 1.41V for battery #6 after five hours of charging, to 1.47V for battery #3 after eight hours of charging.
The pack was then charged for 14+ hours, also at a constant current of 110 mA. The finished pack measured 8.45V after a three-hour rest just before the test. This means that all the batteries were about 1.41V, just like the Yaesu FT-209 battery pack batteries.
Note: This pack was labeled on the curve as Ni-MH but the PB-13 pack is, in fact, a Ni-CD pack.
Figure 12 shows the results of the Kenwood battery pack Test #2 after each cell charge, then pack charged 14+ hours all at 110 mA constant current charging.
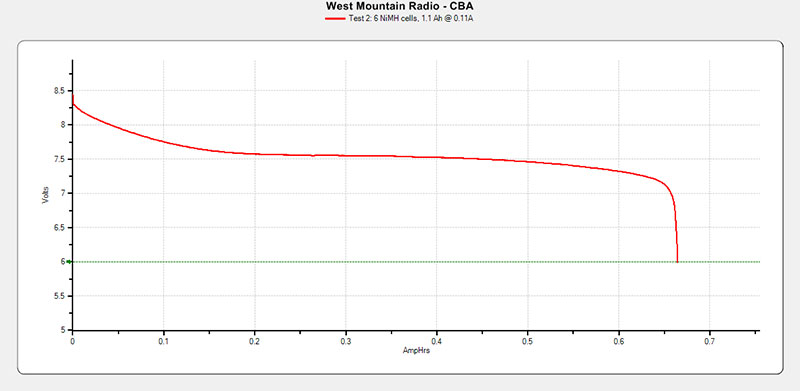
FIGURE 12. Kenwood PB-13 Test 2 results.
Final rating is about 0.65 Ah, 650 mAh, or about 59% of the designed 1.10 Ah, 1100 mAh rating.
For those of us using these battery packs, it really doesn’t matter if there’s a memory effect or non-memory effect failure; the pack no longer works as expected. The big difference is that sometimes a memory effect can be reversed.
If you believe you have a pack exhibiting a memory effect, follow the procedure developed for clearing the effect and see if your pack returns to a more normal discharge capability. You should probably try this method more than once to be sure. If it still doesn’t return the pack to its former glory, then the pack probably has failed due to one of the other failure modes listed previoiusly, or the memory effect can’t be reversed for some other reason.
Klaus and I have had different experiences. Klaus feels sure he and his company really experienced some battery packs with a memory effect. I, on the other hand, have not come across anything like this with my battery packs, or with the ones used in the two companies where I worked, or the more recent packs supplied by members of the Carroll County Amateur Radio Club here in Maryland.
Even the packs recently tested do not fall into the memory effect category because they’re older than three years of service and various discharge/recharge times. The memory effect happens when a pack is constantly discharged and charged within a few percent of being identical every time. Note that even when this is done for more than 700 times, it doesn’t always result in a pack being changed with a memory effect. For a more in-depth explanation, see the article at https://en.wikipedia.org/wiki/Memory_effect.
So, your experiences may be like any of these described or it may be a memory effect. At this point, I’m not sure if there is a really good test (other than trying the restoring procedure for the pack) that can tell us if it was, in fact, a memory effect. Even then, we’ll only know for sure that it was a memory effect if it’s rejuvenated and works correctly from then on.
If you’re like me and you want to avoid this issue completely, then you can always do as I now do: Buy only radios that also have the optional battery case for AA batteries, and then buy good quality NiMH AA batteries and a recharger that charges each cell individually.
By the way, good chargers also have a reconditioning mode to take the batteries down completely and bring them back up slowly. This is the method developed to reform the batteries suffering from the memory effect and it does this for each battery individually.
Lithium Batteries
I received two Lithium battery packs: one was for an HT; the other for a weed whacker/trimmer by Ryobi. My Ryobi electric drill/screwdriver uses the same battery pack. I’ll start with the HT pack shown in Figures 13 through 15.

FIGURE 13. Yaesu FNB-58Li battery pack.

FIGURE 14. Yaesu FNB-58Li battery pack (open).

FIGURE 15. Yaesu FNB-58Li batteries.
This pack when charged and then the pack was tested. Refer to Figure 16.
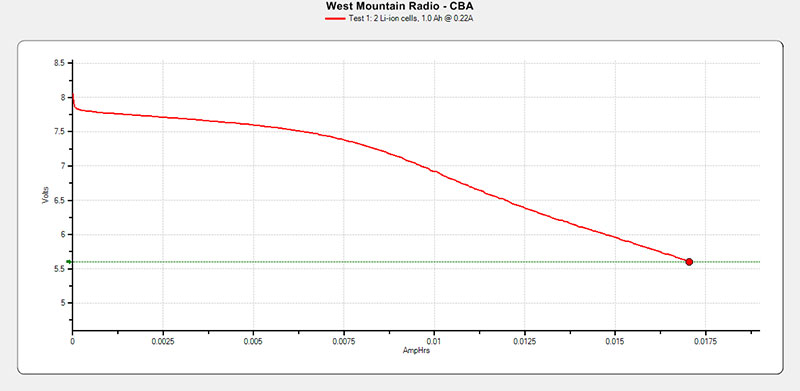
FIGURE 16. Yaesu FNB-58Li test results.
The second test was after each cell was charged individually and then tested individually. Refer to Figure 17.
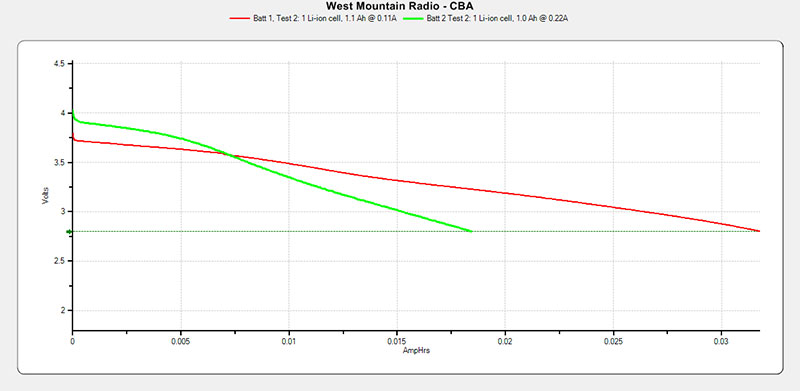
FIGURE 17. Yaesu FNB-58Li additional test results.
In this case, we can see that with battery #2, the green curve (even after individual charge) is just a bit better than half as good as battery #1. Batt 1 ~ 31 mAh and Batt 2 ~ 18 mAh.
The next pack tested was the Ryobi pack, shown in Figures 18 through 21.

FIGURE 18. Ryobi P102 battery back (bottom view).

FIGURE 19. Ryobi P102 battery back (top view).

FIGURE 20. Ryobi P102 battery pack (open).

FIGURE 21. Ryobi P102 batteries.
I didn’t recharge each of these batteries a second time individually because it seemed that they were failing due to old age and frequent use, and not — as far as I could tell — due to a memory effect. All cells were approximately the same voltage. The starting voltage for the pack was ~20.22V; discharged, it was ~16.10V. The discharged batteries individually were measured as:
# Voltage
1 – 3.39V
2 – 3.07V
3 – 3.18V
4 – 3.30V
5 – 3.16V
This pack was the best of all the packs tested in the sense that it was only down to about 50% of its initial designed capacity. The pack was designed to be a 1.3 Ah pack and it delivered ~750 mAh for about 56% of design capacity.
It also looks like the recharging is done for each cell individually, so if you can find a way to remove the 18650 Li cells and reattach the leads, you could actually repair this kind of battery pack. Refer to Figure 22 for the test results.
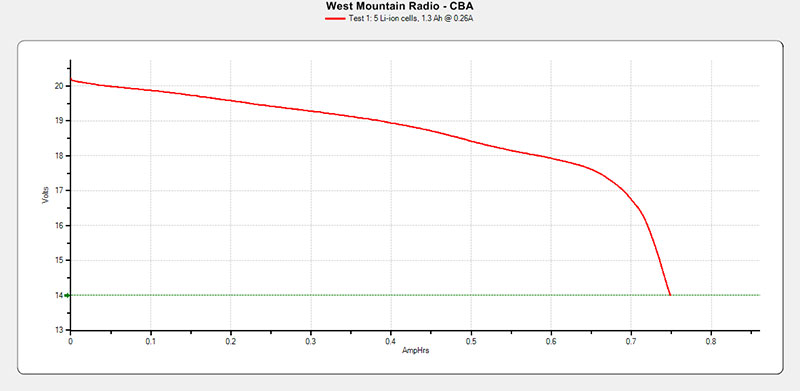
FIGURE 22. Ryobi P102 test results.
Concerning Li battery memory effects, it turns out that these have been observed in Li batteries in some of the newer cars. This doesn’t seem to be holding to what was observed in Ni-Cd or Ni-MH batteries, which require over 700 cycles all discharged and recharges well within + or – 1% to 3% every time.
My point has always been that when using amateur radios or wireless tools, we generally discharge batteries to the recommended discharge point before we recharge them. This means that our tool is slowing down or the radio is not working as it should. Doing this is exactly the wrong thing to do in order to get to the memory effect.
Also, the evidence of my own experience for Ni-Cd batteries has enforced my belief that in normal amateur radio use and electric rechargeable tools, we probably won’t ever see a real memory effect. We’ll probably encounter only things that look like it. (And by probably, I mean something on the order of 0.001% or less probability of ever encountering a real memory effect, i.e., not in my lifetime.)
In the case of the newer cars, are they actually driving these electric cars over 700 times in exactly the same way to have it discharged to the same level within 3% every time? Personally, I find that very difficult to accept. I came across a good article on Li battery memory effect (Memory effect now also found in lithium-ion batteries, by Leonid Leiva, Paul Scherrer Institute; https://phys.org/news/2013-04-memory-effect-lithium-ion-batteries.html) which points out that it’s possible. They do not claim to have forced batteries into exhibiting the memory effect and explain how they managed it.
They only claim that their analysis shows the possibility that it can exhibit in Li batteries and the mechanism that would cause it. Even better, they propose a way to use monitoring software to catch a possible problem and mitigate it.
Another article claims to have caused the memory effect with only one partial charge and discharge cycle. This marks the LiFePO4 anode as the culprit. Here, they claim that only one partial charge and discharge cycle was the cause.
This being the case, I think it means we need to be more concerned with our Li battery health and learn how to insure longevity.
If we use these batteries fully, we’ll probably never see a real memory effect — only things that can look like it. So, for something like your shaver, radio, or tool, never recharge it after every shave, making one QSO, or screwing one screw. Only recharge it when the indicator says to recharge it. Really kind of a no-brainer, don’t you think?
There’s another article to check out. It’s Toyota Confirms: Li-Ion Batteries Have a “Memory Effect” at https://pocketnow.com/li-ion-batteries-memory-effect.
How to Defeat the Memory Effect of Li-Ion Batteries
We’ve all got to realize that we can’t get rid of the memory effect problem. It’s inherent to the technology — and likely to batteries in general. However, now that we know about it, we can adapt and minimize the impact the memory effect has on our electronics.
It’s fairly simple, which may be one reason why it’s not widely adopted. Keep the following rules in mind:
- Realize that your batteries don’t like to live at the very top or the very bottom of their charge capacity. Don’t keep your devices on the charger after they’re fully charged. Similarly, don’t leave them dead in a drawer for (extended) periods of time either.
- Every once in a while, completely discharge your device, then completely charge it up again. Do that a few times in a row. This will help “condition” the battery and will ensure that you get better use of its capacity. Doing this will reduce the rated lifespan of the battery, but it will get you more practical use out of it and prevent a premature death.
That’s it. Pretty simple, right? Ironically, that’s what ham radio operators have been telling us for many decades, and those guys really know their stuff! NV
Battery Failure Mode Quick Reference Section
Here are some common problems which may seem like the memory effect.
One or more weak batteries in the pack.
Test: Measure the voltage of each battery; the weak one(s) will be lower in voltage. Sometimes much lower, but even if at zero volts these batteries can be brought back.
Fix: If caught soon enough, a simple recharge of each cell individually once a year keeps the pack in good working order. A zero volt battery may need a reconditioning charge to bring it back to life.
Note: a) Sometimes a zero volt battery can’t be brought back to life. If this is the case, then see Problem #3 below.
b) Recycle the dead batteries and save the good ones for other projects or recycle them along with the dead one(s).
One or more reversed charged batteries.
The difference is seen when the pack is taken apart and each battery/cell is tested and/or recharged individually. Measure the voltage of each battery; the reversed charged batteries will show negative voltages.
Fix: There is no known fix for a battery with this condition.
Note: Recycle the dead batteries and save the good ones for other projects or recycle them along with the dead one(s).
One or more batteries measuring zero volts and can’t take a charge.
Test: Measure the voltage of each battery and find the zero volt batteries.
Fix: Sometimes this is caused by dendrites — small shorts inside the battery. These can be “blown” away, but in my experience, while it will return the battery to working condition this good state never lasts very long and it ends up not being worth the time and effort.
Note: Recycle the dead batteries and save the good ones for other projects or recycle them along with the dead one(s).
One or more batteries that can take a charge but discharge more quickly than the rest of the batteries in the pack.
Test: Charge each battery individually and then use the pack. If it fails or discharges more quickly than it should, take it apart again and measure each battery. If the same batteries are once again discharged before the others, then these probably suffer from a dried out battery chemistry.
Fix: There is no known fix for these dead batteries.
Note: Recycle the dead batteries and save the good ones for other projects or recycle them along with the dead one(s).
All the batteries can take a charge, but the pack still performs well below the designed mAh rating.
At this point, check the date of manufacture. If it’s more than two years old and has seen a lot of service (or three or more years old with moderate service), the probability is that the batteries are at the end of their service life.
Fix: The pack needs to be replaced.
Fix: If the pack is a year or less old, then it just might be a memory effect. Try the recommended rejuvenation procedure at least twice. If this doesn’t help or if it was older than two years, then replace the pack.























